MSM Studies
What is MSM?
MSM (Methyl Sulfonyl Methane) is a naturally occurring form of organic sulfur, whose chemical formula is CH3SO2CH3. It is the form in which sulfur occurs naturally, within all living organisms, where it acts as a biologically active substance. Methyl Sulfonyl Methane is an odorless, white, crystalline powder that is highly soluble in hot water and in a wide variety of organic solvents.(1) Biologically active organic sulfur has incredible therapeutic and preventive properties. It exhibits such comprehensive medicinal properties, and based on such obvious principles, that its discovery is normally counted among the most important advances that have been made by orthomolecular medicine, in the second half of the twentieth century.
The discovery of MSM
About four decades ago, Dr. Stanley Jacob and Dr. Robert Herschler, two chemists working at the Crown Zellerbach Corporation’s pulp mill, were asked to find a use for lignin, one of the mill’s main waste products. It was thus found that oxidation of lignin in a reactor produced DMSO (dimethyl sulfoxide), a natural form of organic sulfur. This water-soluble compound has a strong, bitter taste and is rapidly absorbed through the skin. Workers who came in contact with the DMSO-containing waste water noticed that as they perspired, they gave off an odor similar to that of DMSO, the bitter taste of which they also felt in their mouths. In addition, these waters seemed to have special therapeutic properties. We still hear many stories about miraculous healings and benefits, but they cannot be proven. It is certain, however, that cuts, scrapes and sprains healed faster when soaked in water containing DMSO. Several workers also noticed that ailments related to arthritis and asthma subsided when they came in contact with this liquid (information provided by George Bergstrom).
Following the original discovery, several articles were published in the United States regarding the therapeutic properties of DMSO, which, however, never reached wide circulation due to its bitter taste and unpleasant odor. DMSO also presented another problem; its topical application could cause skin irritation. For this reason, researchers began to study a derivative of DMSO that could be better tolerated. It was thus observed that oxidation of DMSO produced MSM, a much more stable organic sulfur compound whose medicinal properties were at least equivalent to those of DMSO, with the advantage of being more pleasant to smell and not causing irritation (4, 8, 9).
Natural sources of MSM
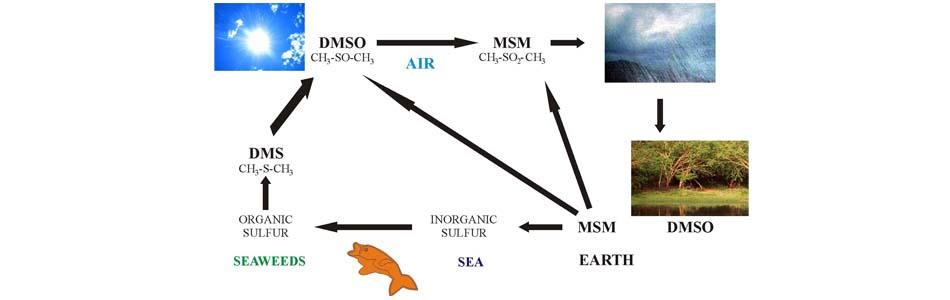
Methyl Sulfonyl Methane is a naturally occurring compound; in fact, it is part of the terrestrial sulfur cycle (3). In the oceans, algae and various forms of plankton absorb large amounts of sulfur from water and convert it into an elemental form with organic bonds. When these algae and planktonic organisms die, their organic molecules are decomposed by enzymatic processes that generate DMS, or dimethyl sulfide, a volatile and poorly water-soluble compound. This is collected in the stratosphere, where, by the action of ultraviolet rays, it is oxidized and transformed first into DMSO (dimethyl sulfoxide), then into MSM (methyl-sulfonyl-methane). DMSO and Methyl Sulfonyl Methane are highly water-soluble and therefore concentrate readily in atmospheric water vapor; through rain, they then return to the earth, where they go on to be an important source of sulfur for plant roots, which absorb them rapidly, storing them in high concentrations. In fact, laboratory research has shown that the concentration of a mixture containing one ppm of DMSO and MSM, with radioactive tracers, can even increase a hundredfold within a few hours within plant roots (4).
This implies that rainwater, in particular, contains MSM in abundance. Large amounts of MSM are also found in fresh fruits and vegetables, in concentrations typically ranging from 1 to 4 mg/kg (3). Fresh, unpasteurized milk from pasture-raised animals contains 2 to 5 mg/kg MSM. Unfortunately, during food preparation, MSM, due to its volatile nature, is quickly lost as a result of cooking processes, or even simply when fruits and vegetables, albeit raw, are not consumed fresh. Pasteurized milk therefore contains less than 0.25 mg/kg MSM, about the same amount found in milk produced by cows raised on dry artificial feed (3). Because of our dietary habits, it is inevitable that modern humans suffer from chronic MSM deficiency.
MSM and human health
The circulatory system of an adult male naturally has MSM levels of about 0.2 mg/kg. Normal adults excrete 4 to 11 mg of MSM daily through urine. Several studies suggest that the systemic concentration of MSM in mammals decreases over the years, perhaps as a result of metabolic changes or changes in dietary habits. According to research findings, there is a minimum blood concentration of MSM, which is necessary for the maintenance of vital functions and tissue defense (8, 9). Low levels of MSM in our bodies are related to unspecified states of fatigue, depression, high sensitivity to physical and mental stress, and numerous degenerative diseases (5, 6). MSM is an important source of sulfur, but it also has unique properties related to its particular chemical composition and biological activities. To understand the preventive and therapeutic properties of MSM, it is necessary to distinguish between “why humans need sulfur” and “why humans need MSM.”